
They offer many advantages over the traditional restriction enzyme cloning we once relied exclusively on.

These newer technologies have become more and more common, and for good reason. See the In-Fusion cloning video tutorial for further details on how SnapGene can simulate Clontech's In-Fusion Cloning while helping you to visualize, control, and document the process.Over the past decade, scientists have developed and fine tuned many different ways to clone DNA fragments which have provided appealing alternatives to restriction enzyme cloning. In the top menu expand Primers and select Export Selected Primers Select the two 'Fragment' primers by clicking their name and holding the Ctrl key during selection. The Rab5FOR primer is a remnant of the restriction cloning where we cloned Rab5 into the pcDNA4_T0 vector.Įxport the primers so you can order them. The 'Fragment' primers (green) are the ones that are used in the In-Fusion cloning. Open the Primers view of the construct (red). Obtain the sequences of the primers Another way of checking the sequences of the primers. Note that these figures are clickable: you can click the primers to view their sequences (green). Open the History view (red) of the construct. Show a graphical overview of the cloning procedure Show a graphical overview of the cloning. Rab5 is nicely fused in frame with AcGFP1. Open the Sequence view of the construct and look at the first line. Open the Product tab and fill in a name for the product at the bottom (red).Ĭlick the Clone button (green) to create the construct. Perform the cloning Create the construct. The red bases are added to create overlaps with the ends of the linearized In-Fusion vector. Here you see the sequences of the primers. Open the Fragment tab and look at the bottom view. If you insert the fragment out-of-frame an error message will appear in the green block at the bottom (red). We'll use the default values for Tm and number of overlapping bases.Ĭlick the Choose Primers button and the cloning product appears. Open the Product tab (green) and click the Choose overlapping PCR primers button (red). SnapGene can design these primers automatically.Īutomatically design primers to amplify Rab5. You do need primers to amplify Rab5 from the mRFP1Rab5 vector creating overlaps with the In-Fusion vector. Both genes are in the same orientation so you do not need to reverse the orientation. Go back to the Map view to see the selection.
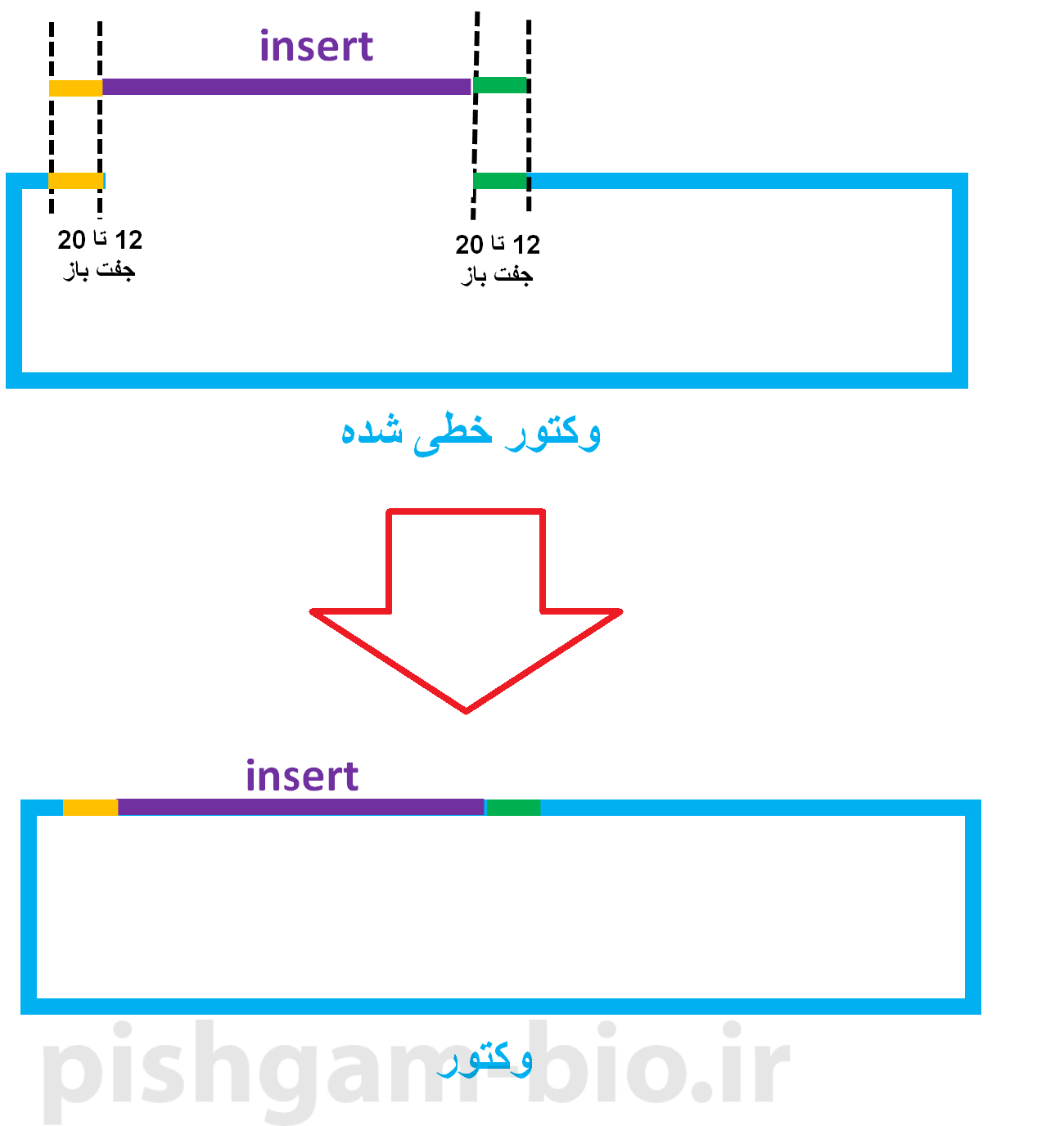

Select the sequence from the start codon to the fifth last base base of the gene (you do not want to include the stop codon in the fusion and you want to insert Rab5 in frame). Open the Sequence view and scroll to the Rab5 gene (1330-1977). Hover your mouse over Rab5 to see its exact location. Open the Fragment tab (red) and select mRFP1Rab5 as the source of the fragment (green). We are going to clone Rab5 into this vector thereby created a N-terminal fusion of Rab5 to AcGFP1. If this was not the case you could linearize the vector by PCR or restriction digest. Since this is an In-Fusion vector it is already linearized. The cloning editor opens on the Vector tab where the vector that was loaded when you opened the cloning editor (pAcGFP1-N In-Fusion® Ready) is opened automatically. Open the cloning editor Open the cloning editorĮxpand Actions in the top menu, select In-Fusion Cloning and then Insert Fragment. 5 Show a graphical overview of the cloning procedure.


 0 kommentar(er)
0 kommentar(er)
